5. Add or remove certification mark
New GTIN for:
Item
Existing higher-level packaging

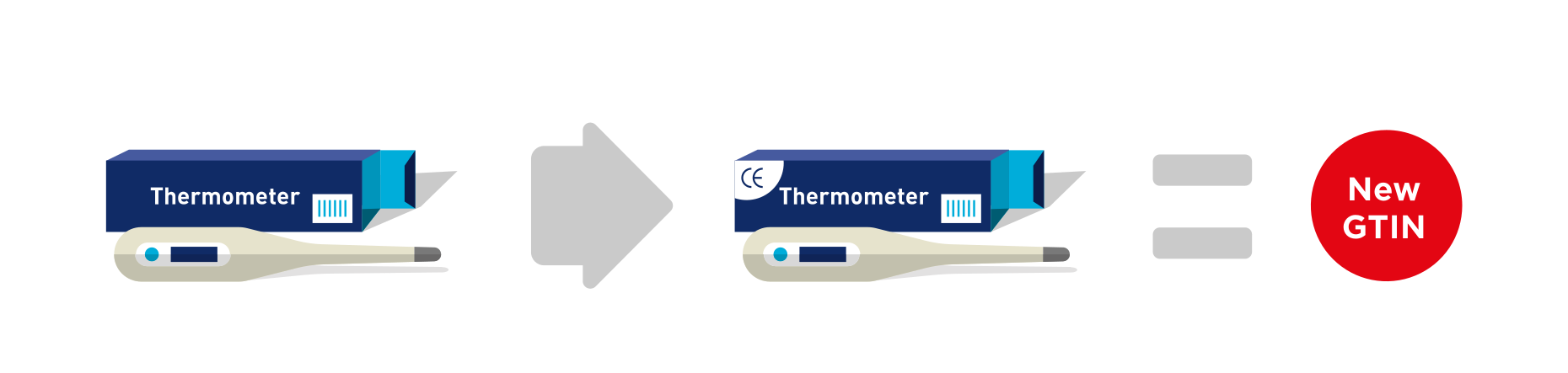
Within the healthcare sector there are many examples of certification marks. A certification mark is a symbol, logo or wording on a product that declares conformance to a regulated set of criteria (e.g., European Certification Mark CE). When a product is changed to include a certification mark (which was not previously shown on the packaging or product itself) a new GTIN should be allocated for markets where the certification mark is of relevance. It is a key principle of GTIN Allocation that the GTIN uniquely identifies the product and its packaging configuration.
A change to packaging to add a new, or remove an existing certification mark (e.g., European Certification Mark CE), that has significance to regulatory bodies, trading partners or to the end consumer, requires assignment of a new GTIN.
New GTIN for:
Item

Existing higher-level packaging

Example business scenarios that require GTIN change
Package:
- A change of a certification mark of one country to that of another.
- Certification Marks that appear, or change, on product labels that impact global distribution channels due to country licence or registration, must be communicated between trading partners and therefore require a GTIN change.
- Removal of a certification mark, for example CE.
Product:
- Addition of an European Certification Mark CE.
- Removal of a CSA certification mark from a product's packaging.
NOTE: However, it should also be noted that when a certification mark is added to enable sales in a new country/market it has no impact on countries/markets where the product was previously sold – in this case there is no need to allocate a new GTIN in the scenario above.
Additional information
- For the purpose of interpretation of this rule, a certification mark is a symbol, logo or wording on a product that declares a product has met specific criteria and standards in formulation, harvesting, processing or manufacturing (e.g., European Certification Mark) and that can be externally verified by a certification authority or agency which can be either a public or private authority.



