Enabling standarisation in the clinical trial supply chain.
What if we all spoke the same language in clinical trials?

Global standards by the industry
The global standards are the result of an industry-led working group that started in 2017, and has included representation from:

Representatives

Clinical trials organisations

Victor Dupouy,
Pfizer and Sanofi

hospitals, IT solution providers,
contract research organisations
...for the industry
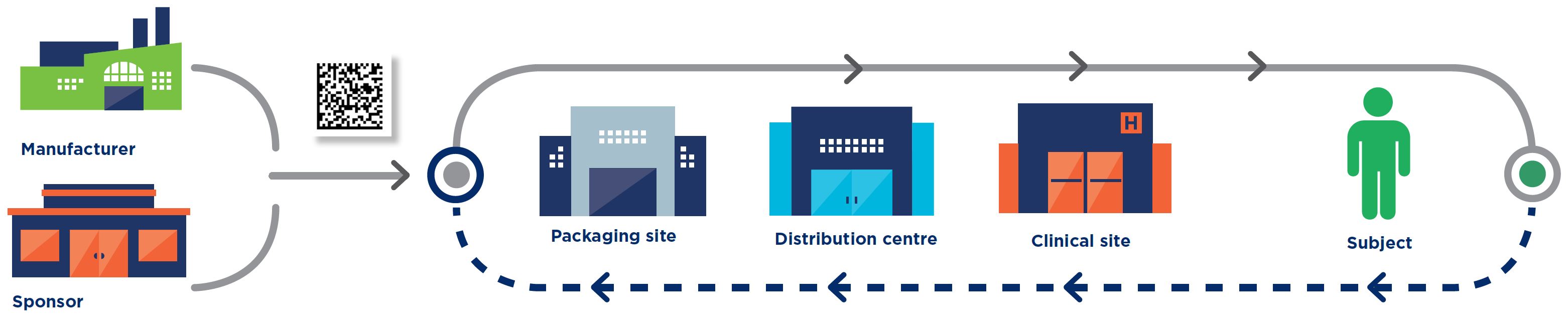

Manufacturer

Sponsor

Packaging site

Distribution centre

Clinical site

Subject
> Applicable to
blinded and unblinded trials
Global Trade Item Number (GTIN)
for unambiguous identification of investigational product kits and their components
Global Location Numbers (GLN)
for unambiguous identification of locations
Standardised GS1 XML messages to communicate about the location, status, availability and dispensing activity of investigational product kits and their components
Across-the-board benefits
Benefits for suppliers
- Quicker data compiling
- Full supply chain traceability enabled
- Fewer transcription errors on the backend
- Less time spent verifying and validating data
Benefits for clinical trials sites
- Saves time
- Improves inventory management
- Limits need for internal re-labelling and transcriptions
- Easy to adopt processes that leverage the barcodes
Benefits for patients
Adopting GS1 Standard adds an element of trust at all levels of the supply chain – a trust that ultimately extends to the patients themselves
What the industry needs is a solution for the identification of investigational products, their locations, and for data interchange in clinical trials. Implementing global standards is a good place to start.
An industry-led effort
57 companies involved in clinical trials have worked together to develop and adopt an identification and barcoding standard for investigational product kits and their components, a global standard for EDI in clinical trials, and to provide guidance about how to identify locations. These standards form the common language all stakeholders can use to communicate across the clinical trial supply chain.
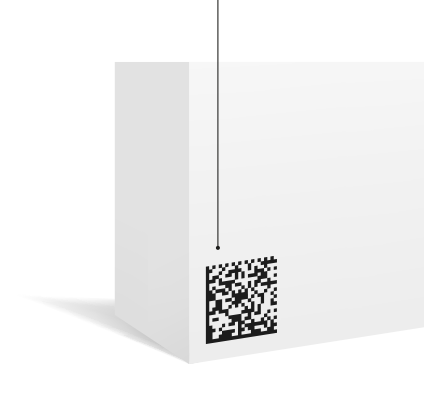
Example of a product with a GS1
DataMatrix barcode
(01)09501101530003 - Global Trade Item Number
(10)AB-123 - Batch Number
(21)000124pc123 - Serial Number
(7240)PR0044 - Protocol Number
(01)09501101530003
(10)AB-123
(21)000124pc123
(7240)PR0044
Global standards unique to clinical trial products
All the standards are intended for use by all parties involved in clinical trial processes, including: manufacturers, sponsors, packaging sites, distributors/3PLs, clinical trial sites, regulators, and patients.
An element of trust
The benefits of employing a standardised approach for labelling investigational products, location identification, and electronic information exchange encompass a wide range of advantages that span across the entire supply chain. These advantages include enhanced accuracy, increased efficiency, familiar processes, and improved safety, resulting in a comprehensive and widespread benefit.
Above all, the incorporation of GS1 Standards in clinical trials instils a crucial sense of trust throughout every aspect of the supply chain, reaching even to the patients themselves. This trust is an invaluable aspect that resonates with all stakeholders involved.
















